Who Should Attend?
This training will benefit professionals in the following industries:
Medical Device, Pharmaceutical, Biotechnology, Food industry.
Course Objective:
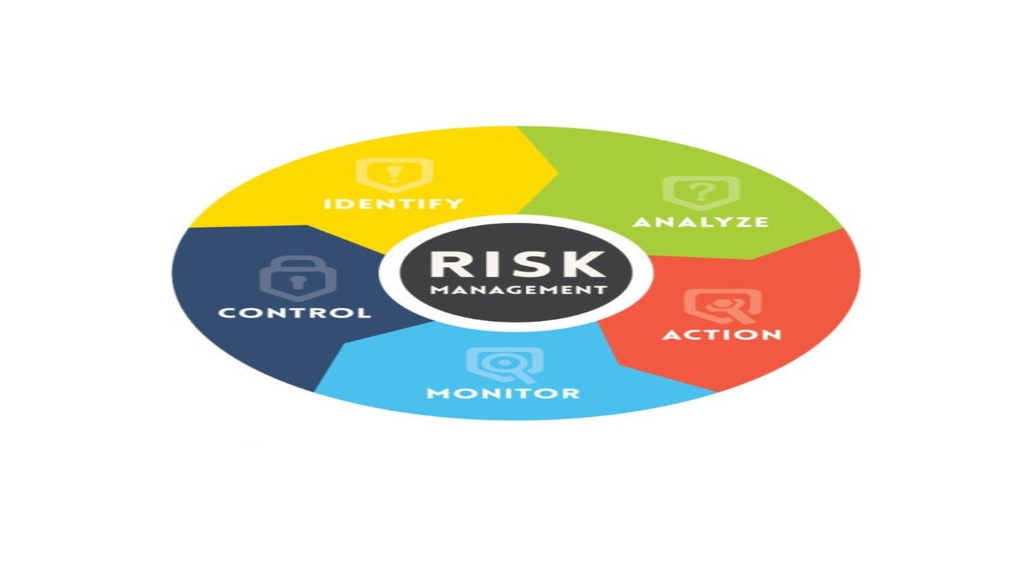
This training is intended to help you better understand and get familiar with best practices for quality risk management (QRM) applicable for pharmaceutical, Medical device& Food industry. This is further intended to discuss how risk management plans can be effectively integrated into a quality system (QS) in the pharmaceutical industry.
quality system (QS) in the pharmaceutical industry.
Course Topics and Number of Sessions :
According to Number of topics chosen from ICH Guideline attached.
New ICH Q9 Requirements
Instructo
SPEAKER
Ahmed Taha Badawi
Risk Management, Quality, cGMP & Sustainable Development Expert